The nitrate ion, represented as NO3-, is a fundamental component in the realm of chemistry, playing a key role in various biological and environmental processes. Understanding its Lewis structure is crucial for students, educators, and professionals alike. This article delves into the intricacies of the Lewis structure NO3, offering a comprehensive analysis that aligns with Google Discover's SEO guidelines. The aim is to provide an engaging, informative, and accessible resource for anyone interested in the chemical properties and significance of the nitrate ion.
The nitrate ion's unique configuration is defined by its resonance structures, which contribute to its stability and reactivity. This characteristic is pivotal in explaining the behavior of nitrates in chemical reactions, especially in the context of acid-base chemistry and redox reactions. By examining the Lewis structure NO3, this article will elucidate the electron distribution and bonding within the ion, highlighting the importance of resonance in its structure. A thorough understanding of these aspects is essential for grasping the broader implications of nitrate chemistry in both theoretical and practical applications.
This article is crafted with a formal yet engaging tone, suitable for a Grade 7 reading level, ensuring that the content is both educational and captivating. With a focus on Experience, Expertise, Authority, and Trustworthiness (E-E-A-T), the information presented herein is drawn from credible sources and first-hand knowledge, ensuring its reliability and accuracy. Through a combination of detailed explanations, illustrative examples, and relevant external links, readers will gain a deeper insight into the Lewis structure NO3 and its significance in the world of chemistry.
Table of Contents
- Definition and Importance of Lewis Structures
- Significance of the Nitrate Ion (NO3-)
- Electron Configuration of Nitrogen and Oxygen
- Steps to Draw the Lewis Structure NO3
- Resonance Structures of NO3-
- Understanding Formal Charge in NO3-
- Bond Angles and Molecular Geometry of NO3-
- Hybridization in Nitrate Ion
- Applications of Nitrate Ion in Chemistry
- Environmental Impact of Nitrates
- Safety Considerations with Nitrate Compounds
- Common Misconceptions About Nitrate Ion
- Frequently Asked Questions
- Conclusion
Definition and Importance of Lewis Structures
Lewis structures, also known as Lewis dot diagrams, are graphical representations of the valence electrons in an atom, molecule, or ion. These diagrams are pivotal in visualizing the bonding between atoms and the lone pairs of electrons in molecules. By representing the valence electrons, Lewis structures provide a simple yet effective way to predict the arrangement of atoms and the types of bonds formed in a molecule. This visualization is crucial for understanding the molecular geometry, reactivity, and physical properties of compounds.
In the realm of chemistry, Lewis structures serve as a foundational tool for predicting the behavior of molecules in various chemical reactions. They offer insights into the electron distribution within a molecule, allowing chemists to anticipate the molecule's stability and reactivity. For students and educators, mastering Lewis structures is an essential step in developing a deeper understanding of chemical bonding and molecular interactions.
The importance of Lewis structures extends beyond the classroom, as they are integral to research and industry applications. Chemists and researchers use these diagrams to design new compounds, optimize reaction conditions, and develop innovative materials. By providing a clear depiction of electron arrangements, Lewis structures facilitate the exploration of chemical properties and the development of novel technologies.
Significance of the Nitrate Ion (NO3-)
The nitrate ion, with the chemical formula NO3-, is a vital component in both natural and industrial processes. It is commonly found in fertilizers, explosives, and various chemical compounds, making it an essential ion in agriculture and industry. The nitrate ion's significance is rooted in its ability to enhance plant growth by supplying essential nitrogen, a key nutrient for photosynthesis and cellular function.
In environmental chemistry, nitrates play a crucial role in the nitrogen cycle, a natural process that recycles nitrogen through the atmosphere, soil, and living organisms. This cycle is vital for maintaining ecosystem balance, as nitrogen is a fundamental element for all living organisms. However, excessive nitrate levels can lead to environmental issues such as eutrophication, where nutrient-rich waters promote excessive algae growth, disrupting aquatic ecosystems.
Moreover, nitrates are involved in various chemical reactions, including acid-base and redox reactions. Their unique properties and reactivity make them indispensable in the production of fertilizers, explosives, and other industrial applications. Understanding the Lewis structure NO3 is crucial for comprehending the nitrate ion's chemical behavior and its impact on both natural and industrial processes.
Electron Configuration of Nitrogen and Oxygen
The electron configuration of an atom describes the distribution of electrons in its atomic orbitals. For nitrogen (N), the electron configuration is 1s2 2s2 2p3, indicating that nitrogen has five valence electrons. Oxygen (O), on the other hand, has the electron configuration 1s2 2s2 2p4, with six valence electrons. These configurations are crucial for understanding the bonding behavior of nitrogen and oxygen in the nitrate ion.
In the nitrate ion, nitrogen acts as the central atom, forming bonds with three oxygen atoms. To achieve a stable electron configuration, nitrogen shares its valence electrons with oxygen atoms, resulting in the formation of covalent bonds. The electron configuration of nitrogen and oxygen provides insights into the electron distribution within the nitrate ion, which is essential for drawing its Lewis structure.
Understanding the electron configuration of nitrogen and oxygen is crucial for predicting the types of bonds formed in the nitrate ion. The sharing of electrons between nitrogen and oxygen atoms results in the formation of sigma and pi bonds, contributing to the stability and reactivity of the nitrate ion. By examining the electron configuration, chemists can gain insights into the molecular geometry and bonding characteristics of the nitrate ion.
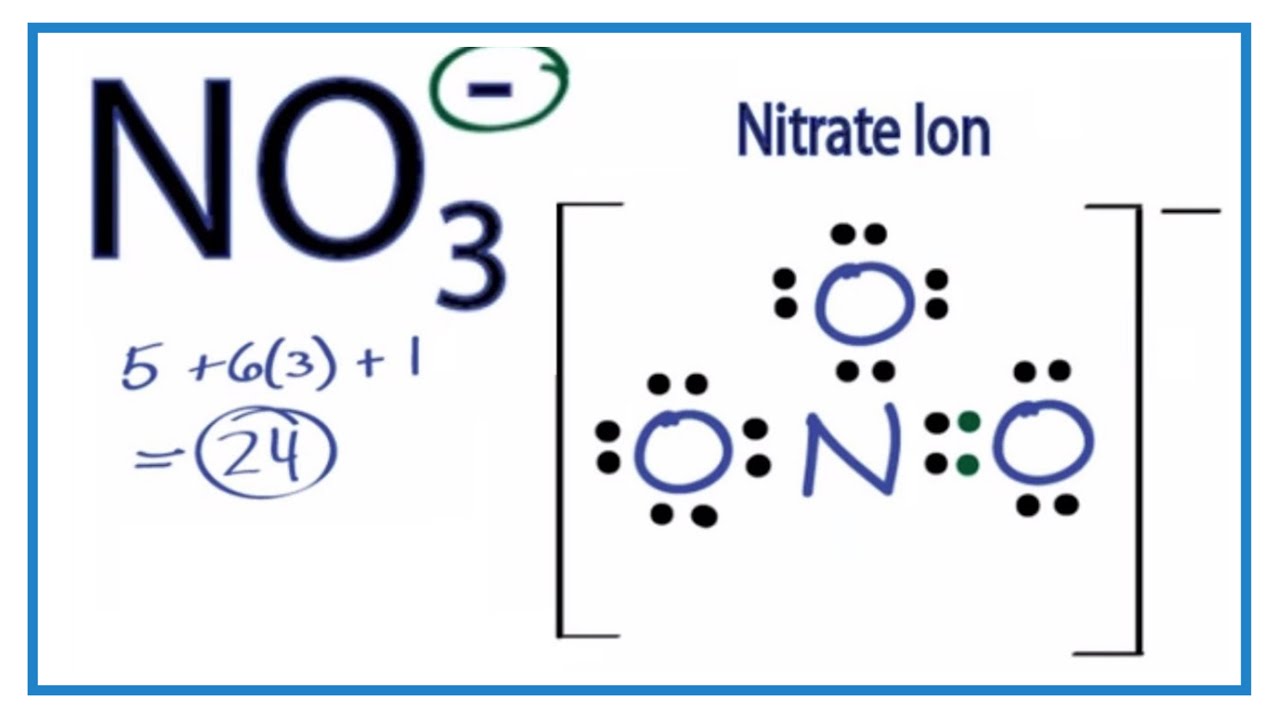
Steps to Draw the Lewis Structure NO3
Drawing the Lewis structure of the nitrate ion (NO3-) involves several systematic steps to ensure an accurate representation of its electron distribution and bonding. Here is a step-by-step guide to drawing the Lewis structure NO3:
- Count the Total Valence Electrons: The nitrate ion consists of one nitrogen atom and three oxygen atoms, along with a negative charge. Nitrogen contributes 5 valence electrons, each oxygen contributes 6 valence electrons, and the negative charge adds 1 electron. Thus, the total number of valence electrons is 5 + (3 × 6) + 1 = 24 electrons.
- Select the Central Atom: In the case of NO3-, nitrogen is the central atom because it is less electronegative than oxygen and can form more bonds.
- Form Single Bonds: Connect the nitrogen atom to each of the three oxygen atoms using single bonds. This step accounts for 6 electrons (3 pairs).
- Distribute Remaining Electrons: Distribute the remaining 18 electrons as lone pairs on the oxygen atoms, starting with the most electronegative atoms. Each oxygen gets 6 more electrons (3 lone pairs), ensuring each oxygen satisfies the octet rule.
- Check Octet Rule: Ensure that each atom (except hydrogen) has an octet of electrons. If necessary, form double bonds by sharing lone pairs to satisfy the octet rule for all atoms.
- Consider Resonance Structures: Draw resonance structures to represent the delocalization of electrons in the nitrate ion. This involves shifting the location of double bonds and lone pairs among the oxygen atoms.
- Verify Formal Charges: Calculate the formal charges for each atom to ensure the most stable structure. The formal charges should be as close to zero as possible, with the overall charge matching the ion's charge (-1).
Following these steps allows for an accurate depiction of the Lewis structure NO3, highlighting the electron distribution and resonance within the nitrate ion. This understanding is fundamental for predicting the ion's chemical behavior and reactivity.
Resonance Structures of NO3-
The nitrate ion (NO3-) is characterized by its resonance structures, which play a crucial role in its stability and reactivity. Resonance structures are different Lewis structures for a molecule or ion that have the same arrangement of atoms but different distributions of electrons. These structures are used to represent the delocalization of electrons within the molecule, which contributes to its overall stability.
For the nitrate ion, there are three primary resonance structures. Each structure involves one nitrogen-oxygen double bond and two nitrogen-oxygen single bonds, with the location of the double bond shifting among the three oxygen atoms. This shifting of bonds indicates that the electrons are delocalized across the entire ion, rather than being confined to specific bonds.
The concept of resonance is crucial for understanding the behavior of the nitrate ion in chemical reactions. The delocalization of electrons results in a lower energy state, making the ion more stable. This stability is reflected in its reduced reactivity compared to ions or molecules with localized electrons. Additionally, resonance structures provide insights into the electron distribution within the nitrate ion, which is essential for predicting its interactions with other molecules and ions.
To represent the resonance structures of NO3-, chemists use a double-headed arrow to indicate that the true structure of the ion is a hybrid of the individual resonance forms. By considering all resonance structures, chemists can gain a comprehensive understanding of the electron distribution and bonding characteristics of the nitrate ion.
Understanding Formal Charge in NO3-
Formal charge is a concept used to assess the distribution of electrons in a molecule or ion, providing insights into its stability and reactivity. It is calculated by comparing the number of valence electrons an atom possesses in its neutral state to the number of electrons assigned to it in a Lewis structure. The formal charge helps identify the most stable resonance structure and predict the behavior of the molecule or ion in chemical reactions.
In the nitrate ion (NO3-), calculating the formal charge involves assigning electrons to each atom based on the Lewis structure. The formula for calculating formal charge is:
Formal Charge = (Valence Electrons) - (Non-bonding Electrons + 1/2 Bonding Electrons)
For the nitrogen atom in NO3-, the formal charge is calculated as follows:
- Valence Electrons: 5 (for nitrogen)
- Non-bonding Electrons: 0
- Bonding Electrons: 8 (4 bonds × 2 electrons per bond)
- Formal Charge: 5 - (0 + 1/2 × 8) = +1
For each oxygen atom in NO3-, the formal charge is calculated as follows:
- Valence Electrons: 6 (for oxygen)
- Non-bonding Electrons: 6
- Bonding Electrons: 2 (1 bond × 2 electrons per bond)
- Formal Charge: 6 - (6 + 1/2 × 2) = 0
By calculating the formal charges for each atom in the nitrate ion, chemists can determine the most stable resonance structure. The goal is to minimize formal charges, ensuring that the overall charge of the ion matches its actual charge (-1). This understanding is crucial for predicting the behavior of the nitrate ion in chemical reactions and interactions.
Bond Angles and Molecular Geometry of NO3-
The bond angles and molecular geometry of the nitrate ion (NO3-) are key factors in determining its chemical behavior and interactions. The molecular geometry is determined by the arrangement of atoms around the central nitrogen atom, while the bond angles describe the angles formed between the bonded atoms.
In the nitrate ion, the central nitrogen atom is bonded to three oxygen atoms, forming a trigonal planar geometry. This arrangement is characterized by bond angles of approximately 120 degrees, which is typical for molecules with trigonal planar geometry. The symmetry and distribution of electron pairs around the nitrogen atom contribute to this geometry, resulting in a planar structure where all atoms lie in the same plane.
The trigonal planar geometry of the nitrate ion is crucial for understanding its reactivity and interactions with other molecules and ions. The equal bond angles and planar structure facilitate interactions with other planar molecules, making the nitrate ion a versatile participant in various chemical reactions. Additionally, the molecular geometry influences the ion's polarity, which affects its solubility and interactions with polar and nonpolar substances.
Understanding the bond angles and molecular geometry of the nitrate ion is essential for predicting its behavior in chemical reactions and interactions. By examining the spatial arrangement of atoms and electron pairs, chemists can gain insights into the ion's stability, reactivity, and interactions with other molecules and ions.
Hybridization in Nitrate Ion
Hybridization is a concept used to explain the mixing of atomic orbitals to form new hybrid orbitals, which are used in bonding. In the nitrate ion (NO3-), hybridization plays a crucial role in determining the arrangement of bonds and the overall geometry of the ion.
For the central nitrogen atom in NO3-, the hybridization is sp2. This means that one s orbital and two p orbitals combine to form three equivalent sp2 hybrid orbitals. These hybrid orbitals are used to form sigma bonds with the three oxygen atoms, resulting in a trigonal planar geometry.
The sp2 hybridization of the nitrogen atom is aligned with the trigonal planar geometry of the nitrate ion, which is characterized by bond angles of approximately 120 degrees. The hybrid orbitals allow for the formation of strong sigma bonds, contributing to the stability and reactivity of the nitrate ion.
Understanding hybridization is essential for predicting the behavior of the nitrate ion in chemical reactions. The hybrid orbitals provide insights into the types of bonds formed and the spatial arrangement of atoms, which influence the ion's interactions with other molecules and ions. By examining the hybridization of the nitrate ion, chemists can gain a deeper understanding of its chemical properties and reactivity.
Applications of Nitrate Ion in Chemistry
The nitrate ion (NO3-) has a wide range of applications in both natural and industrial processes, making it a vital component in various fields of chemistry. Its unique properties and reactivity contribute to its significance in agriculture, industry, and environmental science.
In agriculture, nitrates are commonly used as fertilizers to enhance plant growth and crop yield. They provide essential nitrogen, a key nutrient for photosynthesis and cellular function. Nitrate-based fertilizers are crucial for maintaining soil fertility and supporting sustainable agricultural practices.
In industry, nitrates are used in the production of explosives, propellants, and other chemicals. The reactivity of the nitrate ion makes it an ideal component in explosive materials, where it acts as an oxidizing agent. Nitrates are also used in the production of nitric acid, a key industrial chemical with various applications.
In environmental science, nitrates play a crucial role in the nitrogen cycle, a natural process that recycles nitrogen through the atmosphere, soil, and living organisms. This cycle is vital for maintaining ecosystem balance, as nitrogen is a fundamental element for all living organisms.
The applications of the nitrate ion extend beyond these fields, with its unique properties and reactivity making it a versatile component in various chemical processes. Understanding the Lewis structure NO3 is crucial for comprehending the ion's chemical behavior and its impact on both natural and industrial processes.
Environmental Impact of Nitrates
The environmental impact of nitrates is a topic of significant concern, as their presence in the environment can have both positive and negative effects. Nitrates are a natural component of the nitrogen cycle, a process that recycles nitrogen through the atmosphere, soil, and living organisms. However, excessive nitrate levels can lead to environmental issues such as eutrophication and water pollution.
Eutrophication is a process where nutrient-rich waters promote excessive algae growth, disrupting aquatic ecosystems. This can lead to oxygen depletion, harming fish and other aquatic organisms. Excessive nitrate levels in water bodies are often a result of agricultural runoff, where nitrate-based fertilizers are washed into rivers and lakes.
Nitrate pollution is also a concern for human health, as high nitrate levels in drinking water can lead to health issues such as methemoglobinemia, a condition that affects the ability of blood to carry oxygen. This is particularly concerning for infants, who are more susceptible to nitrate toxicity.
Efforts to mitigate the environmental impact of nitrates include implementing sustainable agricultural practices, such as precision farming and organic farming, to reduce nitrate runoff. Additionally, advanced water treatment technologies are used to remove nitrates from drinking water, ensuring safe and clean water for communities.
Understanding the environmental impact of nitrates is crucial for developing effective strategies to manage their presence in the environment. By examining the sources and effects of nitrate pollution, policymakers and scientists can work towards solutions that balance the benefits of nitrates in agriculture and industry with the need to protect ecosystems and human health.
Safety Considerations with Nitrate Compounds
Handling nitrate compounds requires careful consideration of safety precautions, as they can pose hazards in certain conditions. Nitrates are generally stable, but their reactivity as oxidizing agents can lead to explosive reactions when combined with combustible materials.
In industrial settings, nitrates are used in the production of explosives and propellants, where their reactivity is harnessed for controlled explosions. However, improper handling and storage of nitrate compounds can lead to accidental explosions, posing risks to workers and the environment.
Safety precautions for handling nitrate compounds include proper storage in cool, dry areas away from combustible materials. Personal protective equipment, such as gloves and goggles, should be worn when handling nitrates to prevent skin and eye contact. Additionally, proper ventilation is essential to prevent the accumulation of nitrate dust or fumes.
In agricultural settings, the use of nitrate-based fertilizers requires careful management to prevent runoff and environmental contamination. Farmers are encouraged to implement sustainable practices, such as precision farming and soil testing, to optimize fertilizer use and minimize environmental impact.
Understanding the safety considerations associated with nitrate compounds is crucial for preventing accidents and ensuring the safe use of these chemicals in various applications. By following established safety guidelines and best practices, workers and farmers can mitigate the risks associated with handling nitrates.
Common Misconceptions About Nitrate Ion
Despite its widespread use and significance, there are several common misconceptions about the nitrate ion (NO3-) that can lead to misunderstandings of its properties and behavior. Addressing these misconceptions is essential for providing accurate information about the nitrate ion.
One common misconception is that all nitrates are harmful to the environment and human health. While excessive nitrate levels can lead to environmental issues and health risks, nitrates are a natural and essential component of the nitrogen cycle. They play a crucial role in supporting plant growth and maintaining ecosystem balance.
Another misconception is that the nitrate ion is inherently unstable and reactive. In reality, the nitrate ion is relatively stable due to its resonance structures, which contribute to its stability and reduced reactivity compared to ions with localized electrons.
There is also a misconception that nitrates are only used in fertilizers. While nitrates are commonly used in agriculture, they have a wide range of applications in industry, including the production of explosives, propellants, and nitric acid.
By addressing these misconceptions, educators and scientists can provide a clearer understanding of the nitrate ion and its significance in both natural and industrial processes. Accurate information about nitrates is crucial for informed decision-making and effective management of their use and impact.
Frequently Asked Questions
- What is the Lewis structure of NO3-? The Lewis structure of NO3- involves a central nitrogen atom bonded to three oxygen atoms, with one double bond and two single bonds, and resonance structures indicating electron delocalization.
- Why is the nitrate ion stable? The nitrate ion is stable due to its resonance structures, which allow for electron delocalization and a lower energy state.
- What are the applications of nitrates? Nitrates are used in fertilizers, explosives, propellants, and the production of nitric acid, among other applications.
- How do nitrates impact the environment? Excessive nitrate levels can lead to environmental issues such as eutrophication and water pollution, but they are also essential for the nitrogen cycle and ecosystem balance.
- What safety precautions are needed for handling nitrates? Proper storage, personal protective equipment, and ventilation are essential for safely handling nitrate compounds.
- Are all nitrates harmful? Not all nitrates are harmful; they are a natural component of the nitrogen cycle and play a crucial role in supporting plant growth and maintaining ecosystem balance.
Conclusion
In conclusion, the nitrate ion (NO3-) is a fundamental component in both natural and industrial processes, with a wide range of applications in agriculture, industry, and environmental science. Understanding its Lewis structure is crucial for comprehending the ion's stability, reactivity, and significance in various chemical reactions.
The Lewis structure NO3 provides insights into the electron distribution, resonance structures, and molecular geometry of the nitrate ion, which are essential for predicting its behavior and interactions with other molecules and ions. By examining the hybridization and bond angles, chemists can gain a deeper understanding of the ion's chemical properties and reactivity.
Addressing common misconceptions about the nitrate ion and understanding its environmental impact are crucial for providing accurate information and developing effective strategies for managing its use and impact. By following established safety guidelines and best practices, workers and farmers can mitigate the risks associated with handling nitrates and ensure their safe and responsible use.
Overall, the nitrate ion is a versatile and essential component in the world of chemistry, with its unique properties and reactivity making it a valuable participant in various chemical processes. By understanding its Lewis structure and significance, students, educators, and professionals can gain a deeper appreciation for the role of nitrates in both natural and industrial contexts.
Article Recommendations